Polaris Quantum Biotech (PolarisQB) has released a comparative study indicating that its Quantum-Aided Drug Design (QuADD) platform, powered by D-Wave Quantum Inc.’s annealing technology, significantly outperforms classical Generative AI in lead identification for drug discovery. The study, currently a preprint on ChemRxiv, compared QuADD to the Bond and Interaction generating Diffusion model (BInD), a representative AI-based diffusion algorithm. The findings suggest that quantum annealing can reduce the timeline for selecting pre-clinical molecular candidates from months to hours while delivering higher-quality results.
The QuADD platform utilizes the D-Wave Advantage system, featuring over 5,000 qubits, to solve complex combinatorial optimization problems. By framing drug design as a Quadratic Unconstrained Binary Optimization (QUBO) problem—specifically an adaptation of the “Knapsack Problem”—QuADD explores a theoretical chemical space of up to 10³⁰ molecules. This approach allows for the simultaneous optimization of multiple properties, including binding affinity, synthetic complexity, metabolic stability, and toxicity.
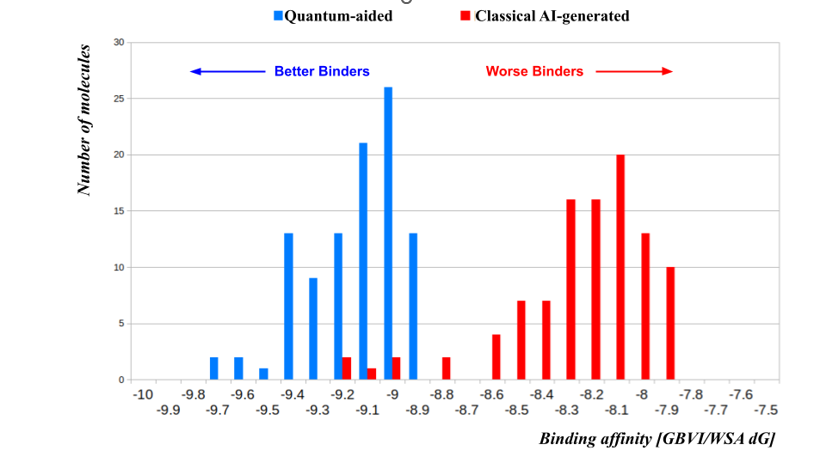
Key Comparative Results: Efficiency and Quality
The study utilized Thrombin, a blood-clotting enzyme, as a case study to design over 3,000 molecules for comparison. The results highlighted a dramatic difference in computational efficiency and pharmacological relevance:
- Computational Speed: QuADD required approximately 30 minutes to generate 3,000 molecular candidates. In contrast, the BInD AI model required 40 hours on a node equipped with a single NVIDIA GPU to produce an equivalent set.
- Binding Affinity: The top 100 QuADD-generated molecules showed substantially stronger predicted binding affinities, improving scores by at least 1 kcal/mol—representing an order of magnitude improvement in predicted binding strength over the AI-generated candidates.
- Synthesizability: While the AI model produced a more diverse set of “creative” molecular scaffolds, many failed essential drug-likeness filters or were synthetically infeasible. QuADD’s candidates exhibited lower synthetic complexity, making them more actionable for experimental testing.
Commercial and Strategic Impact
The ability to triage large chemical spaces rapidly and effectively has immediate implications for the pharmaceutical and biotech industries. Auransa, a clinical-stage biopharma company, is already implementing QuADD to design compounds for challenging binding pockets. By providing a “correct-first” approach to molecular generation, PolarisQB aims to reduce the high attrition rates typically seen in the early stages of drug discovery, where many classical AI leads fail during laboratory validation.
Read the full blog summary from Polaris Quantum Biotech here and the technical preprint on ChemRxiv here.
January 12, 2026